Paperless Validation in the Pharmaceutical Industry
In the regulated industries there is the saying “what is not documented is not done”. And in numerous regulated industry organizations, the de facto method of documenting the work being done is almost always paper or tools made . The lack of acceptable alternative technologies and strict governmental control and regulations have not helped in festering the enthusiasm and environment required for moving away from the present paper-based situation.
So what can be done for this and how can you transform your workplace from the traditional ways of old, to the digital future rising on the horizon?
So what can be done for this and how can you transform your workplace from the traditional ways of old, to the digital future rising on the horizon?
What is paperless validation anyway?
Before going into the topic of paperless validation in the pharma industry, it's important to go over the basics of Good Documentation Practice (GDocP). Data integrity is the cornerstone of GDocP in any kind of records - paper or electronic.
The ALCOA+ acronym refers to a collection of data integrity principles. ALCOA stands for the five original principles of data integrity which are Attributable, Legible, Contemporaneous, Original and Accurate.
These five principles have since been updated to ALCOA+, which adds the following additional four principles: Complete, Consistent, Enduring and Available. The FDA introduced these principles and continues to use it to this day.
While the principles of ALCOA+ will not be discussed in length in this article, it is vital to describe them as a prelude to understanding and discussing paperless validation.
The ALCOA+ acronym refers to a collection of data integrity principles. ALCOA stands for the five original principles of data integrity which are Attributable, Legible, Contemporaneous, Original and Accurate.
These five principles have since been updated to ALCOA+, which adds the following additional four principles: Complete, Consistent, Enduring and Available. The FDA introduced these principles and continues to use it to this day.
While the principles of ALCOA+ will not be discussed in length in this article, it is vital to describe them as a prelude to understanding and discussing paperless validation.
Drivers behind a paperless validation solution
As systems, solutions and therefore corresponding documentation nowadays is becoming increasingly complex. This fact presents documentation with two important challenges: on one hand we have the need for more efficient and leaner means, while on the other there is always the need to remain compliant to the regulatory requirements and expectations. The struggle to generate and maintain complex documentation throughout the lifecycle of a system is very real. Among them we identify:
• Document generation and updates are labor intensive
• Document quality and formatting requires additional work
• Documentation reviews and ensuring repeated data is consistent and accurate across different documents
• Data integrity
• Change Management
To meet the demand of a leaner, more efficient, and compliant validation execution, a compliant database solution which utilizes a document-centric approach and provides all the benefits of robust data management in a familiar, tested and approved documentation environment is required.
And this is where the paperless validation solution comes in!
• Document generation and updates are labor intensive
• Document quality and formatting requires additional work
• Documentation reviews and ensuring repeated data is consistent and accurate across different documents
• Data integrity
• Change Management
To meet the demand of a leaner, more efficient, and compliant validation execution, a compliant database solution which utilizes a document-centric approach and provides all the benefits of robust data management in a familiar, tested and approved documentation environment is required.
And this is where the paperless validation solution comes in!
Paper-based versus paperless validation approach
The reduction in, and ideally removal of, paper-based validation documentation is an immediate and obvious benefit of paperless validation.
Take a look at the following traditional approach, in which some paper-based activities are necessary at each phase.
Take a look at the following traditional approach, in which some paper-based activities are necessary at each phase.
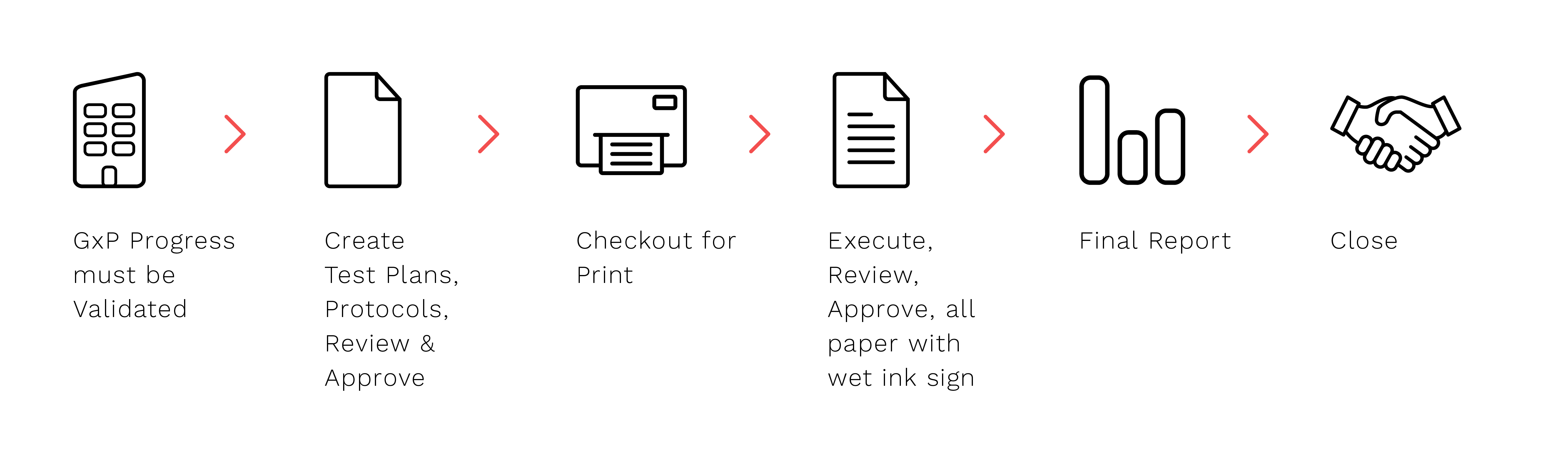
When using a paper-based approach, the reviewers may want to print off the draft document, provide their remarks on it, and return it to the author for incorporation, and then go through the process again for wet-signature pre-approval.
The amount of printing paper wasted, the time it takes to track the movement of documents at various stages, including the physical effort required to move them, and the time it takes to track down reviewers/approvers all add up to resources (time and money) wasted – an unavoidable waste in a paper-based approach.
With a 21 CFR Part 11 compliant paperless solution, the review and pre-approval process could be done electronically including tracking for project management – resulting in immediate cost savings!
The amount of printing paper wasted, the time it takes to track the movement of documents at various stages, including the physical effort required to move them, and the time it takes to track down reviewers/approvers all add up to resources (time and money) wasted – an unavoidable waste in a paper-based approach.
With a 21 CFR Part 11 compliant paperless solution, the review and pre-approval process could be done electronically including tracking for project management – resulting in immediate cost savings!
Documentation Status
When it comes to executing pre-approved test plans on paper, the handwritten document(s) are subjected to different Good Documentation Practice (GDP) and Data Integrity (DI) concerns, whether deliberate or not.
Entries must been tracked with a full audit trail and electronic signature in a purposely developed 21 CFR Part 11 compliant paperless solution.
Cross-referencing the URS points in the execution documents could lead to the creation of an automated created and maintained Requirement Traceability Matrix (RTM).
It's easy to overlook the fact that paperless validation is a controlled computerized system in and of itself.
Entries must been tracked with a full audit trail and electronic signature in a purposely developed 21 CFR Part 11 compliant paperless solution.
Cross-referencing the URS points in the execution documents could lead to the creation of an automated created and maintained Requirement Traceability Matrix (RTM).
It's easy to overlook the fact that paperless validation is a controlled computerized system in and of itself.
Challenges of the paperless validation solution
Implementing a paperless validation solution isn't a silver bullet for a poor documentation procedure or a poorly thought-out business process.
The chosen paperless validation solution is a tool that can help with ensuring consistency in approach (harmonization, templates, etc.) and having design features built into the application to help as much as possible with document preparation and execution, ensuring that they are better managed and thus better for retrieval for any reason and/or purpose.
Secondly, top-tier management must commit to leading the organization away from the "we've always done it this way" approach and encouraging employees to adopt a better way of doing things. As said briefly in the above paragraph, a paperless validation solution isn't a silver bullet, and no single solution is flawless. However, if the "fit for intended use" paperless validation solution is chosen using the same thought process as equipment qualification – that is, defining the User Requirement Specification (URS) for the paperless validation solution – the cost-benefit analysis will almost certainly point to a cost savings.
Lastly, one additional challenge would be for an established organization to initiate the transition from a paper-based to a paperless validation system. Starting small and structuring a "Proof of Concept" with the paperless validation solution vendor allows for a controlled transition by applying the lessons learned when scaling up or out. This culture could be gradually introduced, and competency built in this manner. Keep in mind that Rome was not constructed in a day.
The chosen paperless validation solution is a tool that can help with ensuring consistency in approach (harmonization, templates, etc.) and having design features built into the application to help as much as possible with document preparation and execution, ensuring that they are better managed and thus better for retrieval for any reason and/or purpose.
Secondly, top-tier management must commit to leading the organization away from the "we've always done it this way" approach and encouraging employees to adopt a better way of doing things. As said briefly in the above paragraph, a paperless validation solution isn't a silver bullet, and no single solution is flawless. However, if the "fit for intended use" paperless validation solution is chosen using the same thought process as equipment qualification – that is, defining the User Requirement Specification (URS) for the paperless validation solution – the cost-benefit analysis will almost certainly point to a cost savings.
Lastly, one additional challenge would be for an established organization to initiate the transition from a paper-based to a paperless validation system. Starting small and structuring a "Proof of Concept" with the paperless validation solution vendor allows for a controlled transition by applying the lessons learned when scaling up or out. This culture could be gradually introduced, and competency built in this manner. Keep in mind that Rome was not constructed in a day.
Final thoughts
There's no doubting that as documentation becomes more complicated, methods to achieve more efficient and leaner execution than a compliance database system that takes a document-centric approach will be required. Choosing the correct paperless solution vendor with an established track record can aid in the technology's effective adoption, replacing the paper-based approach's outgoing weight. The road to full acceptance of paperless validation will be rocky, but if you stick with it, you'll save tenfold!
Finally, we leave you with some crucial aspects and thought-provoking questions to consider when choosing a paperless validation solution:
• How compliant is the paperless validation solution to i.e. FDA 21 CFR Part 11 and Eudralex Volume 4 Annex 11 including user management, audit trail, and others?
• What is the limitation in its scalability across multiple sites?
• What is the vendor experience in a GMP regulated environment?
• Does the paperless validation solution have the capability of progress reporting in real-time i.e. document status against the project, system owner, etc?
• Does the paperless validation solution have the capability to ensure harmonization of the approach and what is the control over it?
• Does it have review and approval functionality built-in?
• Does it have an electronic test-execution process?
• Does it auto-generate validation summary reports?
• Will it offer validation storage, search, and retrieval?
At i2b we can help you with deployment, validation and provide ongoing support for the leading paperless validation solutions, whether it is a single process at one site, or involves large scale deployments covering multiple sites and numerous users.
Reach out to us for a full assessment of your requirements and a full risk-based estimation of the impact to your business operations!
John Fenakis – Aug / 2021
Finally, we leave you with some crucial aspects and thought-provoking questions to consider when choosing a paperless validation solution:
• How compliant is the paperless validation solution to i.e. FDA 21 CFR Part 11 and Eudralex Volume 4 Annex 11 including user management, audit trail, and others?
• What is the limitation in its scalability across multiple sites?
• What is the vendor experience in a GMP regulated environment?
• Does the paperless validation solution have the capability of progress reporting in real-time i.e. document status against the project, system owner, etc?
• Does the paperless validation solution have the capability to ensure harmonization of the approach and what is the control over it?
• Does it have review and approval functionality built-in?
• Does it have an electronic test-execution process?
• Does it auto-generate validation summary reports?
• Will it offer validation storage, search, and retrieval?
At i2b we can help you with deployment, validation and provide ongoing support for the leading paperless validation solutions, whether it is a single process at one site, or involves large scale deployments covering multiple sites and numerous users.
Reach out to us for a full assessment of your requirements and a full risk-based estimation of the impact to your business operations!
John Fenakis – Aug / 2021
i(nsight)2b
i(nsight)2b will bring you insights and news about implementation strategies, CSV methodologies and interesting insights around new technologies, platfroms and more. Here we will share our latest insights from within the Pharma, Biotech, and IT industries.
Rethink-TMRW_Insight
Rethink: TMRW
An annual summit about the future of track and trace solutions and holistic digital supply chains. Powered by our partner Movilitas we support this conference 10-11 Oct 2022 in Frankfur...
Read MoreGxP and Scrum Insight
GxP and Scrum in the Pharmaceutical Industry
In the pharmaceutical industry, which is highly regulated, strict rules apply in all its sectors, including the computer system projects. Such rules are ...
Read MoreComputer System Validation and Climate Change
Recently, many businesses have made serious commitments to address their own actions to contribute to climate change. Focusing on sustainability in business models and corporate governance can give bu...
Read MoreValidation for the Cloud Insight
The pharmaceutical sector's information technology requirements are following industry trends, forcing a transition to cloud computing. But how does this affect your validation efforts? Click here to
...
Read MoreThe Pharma Industry is turning to blockchain technology – are you prepared?
We covered the recent emergence of Blockchain technology as well as the challenges it presents to the validation activities, but can this technology transform a more “traditional” organization like a ...
Read Moreinterviewing2b: Regular V Model Computer System Validation, is this still feasible?
Computer system validation has been getting increasingly complex and with a growing number of details required, while often the validation effort overshoots the implementation or development work spen...
Read MoreBlockchain technology & Computer System Validation – what is the impact?
Blockchain technology was introduced over a decade ago, and now it is not only used for cryptocurrency platforms, but it has also evolved into something more significant with the ability to change the...
Read MorePaperless Computer System Validation (CSV)
In the regulated industries there is the saying “what is not documented is not done”. And in numerous regulated industry organizations, the de facto method of documenting the work being done is almost...
Read More