GxP and Scrum in the Pharmaceutical Industry
In the pharmaceutical industry, which is highly regulated, strict rules apply in all its sectors, including the computer system projects. Such rules are GMP, which outline quality standards and manufacturing guidelines.
Meanwhile, software teams have been benefitting from agile development methods, such as Scrum. Several companies in the pharmaceutical industry have adopted agile practices, while keeping development in compliance with regulations, but conflicts arise and decisions must be made in favor of agility or formality.
So, how can Scrum be used in a GxP environment, and what are the benefits from using Scrum in your regulated / GxP company?GxP Compliance
Compliance with manufacturing regulations (GMP/GxP guidelines) ensures that medicinal products meet safety, quality, and efficacy standards; provides patients with beneficial pharmaceutical treatment options, without causing preventable harm; ensures that information published by manufacturers and distributors is accurate (e.g. evidence-based dosing recommendations, safety risks, drug-to-drug interaction warnings, etc.).
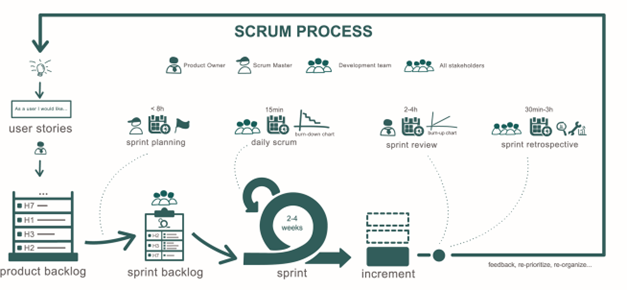
Scrum as part of Agile Methodology
Agile software development is based on an incremental, iterative approach. Instead of in-depth planning at the beginning of the project, Agile methodology is open to changing requirements over time and encourages constant feedback from the end users.
The Scrum project management method is a type of Agile methodology, that employs an iterative, incremental approach to optimize predictability and to control risk. Scrum engages groups of people who collectively have all the skills and expertise to do the work and share or acquire such skills as needed.
The main control mechanism used in Scrum methodology is to break the development up into several Sprints. Each Sprint aims either to deliver a well-defined subset of the overall required functionality or to perform well-defined restructuring of the architecture of the system. This is known as refactoring in agile terminology.
Challenges of using Scrum in a GxP environment
If this approach is not carefully managed, it could degenerate into a process with all the problems that the Waterfall method was designed to avoid, so various means are used to avoid this. In fact, the Scrum methodology, as do all agile approaches, often requires more discipline than the Waterfall method.
Furthermore, continuous iterations, where all artifacts change and evolve, put enormous pressure on the development team and challenge its capacity to produce working software consistently. In regulated environments, the traceability chain that links requirements, architecture, design, implementation and testing, as well as the control of changes to any of the former, is a tough expectation to fulfill.
Also, requirements, architecture, detailed design and test procedures are evolving throughout development, but static formal documents are also needed to satisfy the auditors' expectation. It becomes a daunting task when they evolve and change at the rhythm of every iteration, making it very hard to produce up-to-date documents that make sense, when inspected by an auditor.
Final thoughts
Undoubtedly, using Agile methodology like Scrum is very appealing and efficient in today’s constantly changing technology world. But in the GxP world, it is mandatory to have those aspects in place that will maintain the compliance of the implemented system. Therefore, it is crucial to choose the correct validation solution when combining agility with compliance.
At i2b we can help you with deployment, validation and provide on-going support for agile method validation for GxP-related project, whether it is a single process at one site, or involves large-scale deployments, covering multiple sites and numerous users.
Reach out to us for a full assessment of your requirements and a full risk-based estimation of the impact to your business operations!